MSK Innovation Accelerator
No longer accepting applications
Accelerating the development of musculoskeletal innovations to create and scale early-stage MSK ventures across the UK.
How it works
A commercialisation roadmap supported by global experts
Supported by 
ORUK is one of the most significant funders of MSK research in the UK. It is also the first MSK charity to actively support start-ups and entrepreneurs. Since 2004 it has invested almost £12m on projects that expand knowledge, improve patient outcomes and pioneer new forms of diagnosis and treatment.
Roadmap your path to commercialisation
Gain the insight and knowledge to develop a roadmap to guide you through the complex journey of product development through to market. This will help you understand where your time and budget need to be focussed, how to structure your MSK venture, how you can set appropriate goals and more importantly, how you can achieve them.
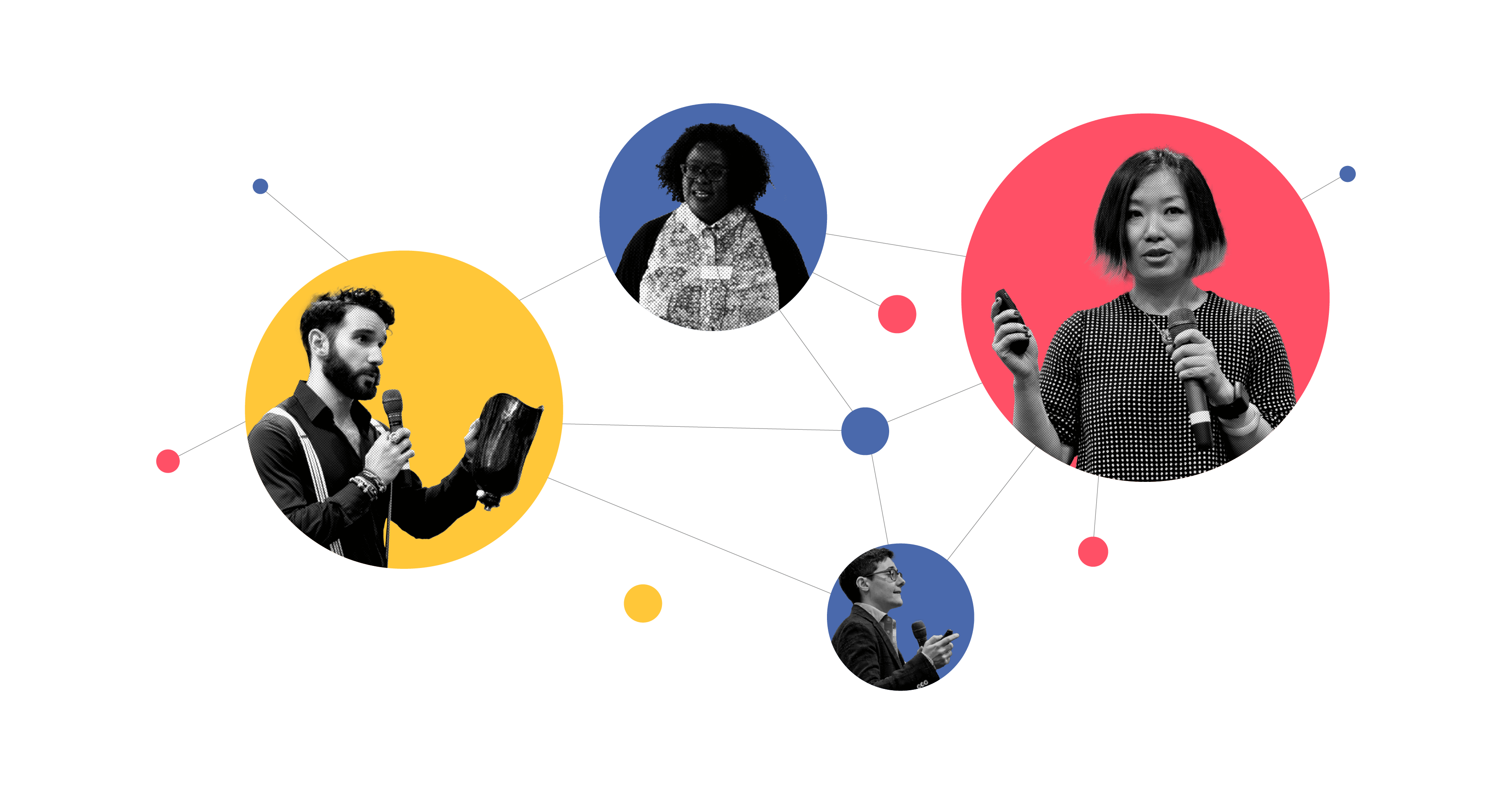
Expand Your Network
You can’t make this journey alone. Our programme will introduce you to individuals and networks with the knowledge and keys to doors that are notoriously difficult to find and open. Expanding your network is a key step to getting to market successfully.
Investment and Growth Opportunities
At the end of the accelerator you will have the opportunity to pitch your venture to the ORUK Investment Committee which could result in up to a £100k investment by ORUK.
The results
Programme Outcomes
Starting with an immersive in person bootcamp followed by a series of online masterclasses, participants will cover the foundations of entrepreneurship, venture building, evidence and evaluation for market access and regulatory considerations. In addition to this, participants will receive support from subject matter experts who will help in navigating their routes to commercialisation.
Participants can expect to gain a greater understanding of effective and applied use of start-up methodologies ie. Lean Startup, Business Model Canvas, Value Proposition Canvas.
Defined Target Market: Understand how innovations are adopted into marketplaces, including the NHS and international & private sector organisations.
Communicating to Key Stakeholders: Gain the skills to build successful relationships with key stakeholders and partner networks to progress your venture, and refine your pitch to successfully communicate your venture to early-stage funders.
Commercialisation Pathway: Prepare your MedTech venture for commercialisation through developing market access and valuation strategies, and understand how to adapt these strategies.
What are the eligibility criteria?
What are the key dates?
The programme will run from late autumn 2023 until spring 2024.
Application period: 11 August – 15 October 2023
Interviews with shortlisted applicants (virtual): 06-07 November 2023
Programme kick-off (in-person, London): late November 2023
Mid-programme check-in (in-person, London): April 2023
Pitching to ORUK Investment Committee: Spring 2024
Dates are to be confirmed but successful applicants should be prepared for an in person kick-off in London in late November 2023. Please continue to visit this page for updates.
Do I need to be incorporated to apply for the programme?
No, you do not need to have an incorporated company in order to apply. However, you must be incorporated to be considered for investment by ORUK.
Is this a full-time programme?
The programme will be delivered over 6 months and will be hybrid in its delivery, with the only mandatory in-person events being the kick-off and mid-programme check-in. In addition to masterclasses and 1:1 sessions, there will be a digital learning component that includes 3 modules to complete in your own time before the conclusion of the programme.
To make the most of the programme we suggest dedicating between 2-5 hours per week to the content (masterclasses, 1:1 sessions, digital learning).
Will I get an investment by ORUK at the end of the programme?
Investments made by the ORUK Investment Committee will be determined on a case-by-case basis, subject to ORUK’s funding terms and conditions.
Make it happen
Register your interest
Collaborate with us to create a healthier future.
MTSC is uniquely placed to facilitate exchange between MedTech innovators and industry players and help them succeed in a rapidly changing and competitive landscape. Our innovation support ecosystem creates value through world-class entrepreneurship training, access to funding, mentorship and expertise.
For more information or to partner with us, please get in touch.

